The Potentiostat
The potentiostat is what controls the electrode potential during a CV. The potentiostat can also perform a wide variety of other tasks that might interest the reader, including constant current experiments, constant potential (bulk) electrolyses, potential step chronocoulometry, and impedance spectroscopy, but cyclic voltammetry is extremely useful and can often be used to obtain the same information as other techniques with less effort. Modern potentiostats are controlled by computer software and it is important to become familiar with the software that is specific to your potentiostat. The leads (wires that connect the electrodes to the potentiostat) are usually color coded for the type of electrode that they are meant to connect with; it is important to connect each electrode to the correct lead. In order to collect a simple CV, one must know how to: connect the electrodes in the appropriate manner, measure the open circuit (rest) potential of the electrode, measure and compensate for the solution resistance, and inform the potentiostat of the parameters needed to perform the desired experiment. The directions for completing the aforementioned tasks should be contained within the operating manual that accompanies the specific potentiostat and software.



hover over pic for description
The Cell
The container where the experiment takes place is called “the cell.” The cell can be any container, but it is commonly made of glass. Cells can come in many shapes and with many accessories. Common shapes are commercially available, but they can also be made to custom specifications by a scientific glassblower. Most CVs can be collected in simple cells that have a single compartment to hold the electrolyte solution and electrodes.



hover over pic for description
The cell should be as clean as possible. Special care should be taken to remove any alkali or halide salts, water (for non-aqueous electrochemistry), organic molecules from cleaning solvents (ethanol, acetone), and trace transition metals from the surface of the cell before use. Sufficiently cleaned glassware for aqueous electrochemistry can be prepared by soaking the glass in nitric acid or aqua regia (a 3:7 mixture of concentrated hydrochloric acid and concentrated nitric acid) for a few hours, followed by washing with deionized water, and storing upside-down or covered to prevent dust or other particulate matter from finding its way into the cell. If one is performing non-aqueous electrochemistry, additional washing with ethanol or acetone, followed by oven drying for a few hours should be employed to ensure the absence of water.

hover over pic for description
Controlling the composition of the headspace above the analyte solution is useful if you want to remove O2, as it can react with certain analytes or get reduced itself, or introduce a gas like CO2 or H2 which may be a substrate that you want to try to transform through electrocatalysis. One can saturate the analyte solution with a gas of choice sparging the solution with gas flowing from a needle or tube for several minutes. If the CV is being collected in a volatile solvent, the gas should be pre-saturated with the solvent before it enters the cell to ensure that the volume does not change during the course of the experiment, which can be accomplished with a gas sparger.

hover over pic for description
In order to ensure that the analyte solution remains saturated, it is important to cover it with a layer of the gas of interest. This can be accomplished by placing some kind of covering over the top of the cell and inserting a tube or needle so that a positive pressure of the gas is always maintained. Commercially available cells are available with tight-fitting teflon tops with room for the electrodes and a hole through which a gas inlet can be inserted, but Parafilm also works. If a strong gas stream is too close to the surface of the solution and perturbs it in any way, strange features will be observed in the CV and incorrect data will result. CVs require the solution to be still, and any gas streams introduced into the cell should be as far away from the solution as possible.
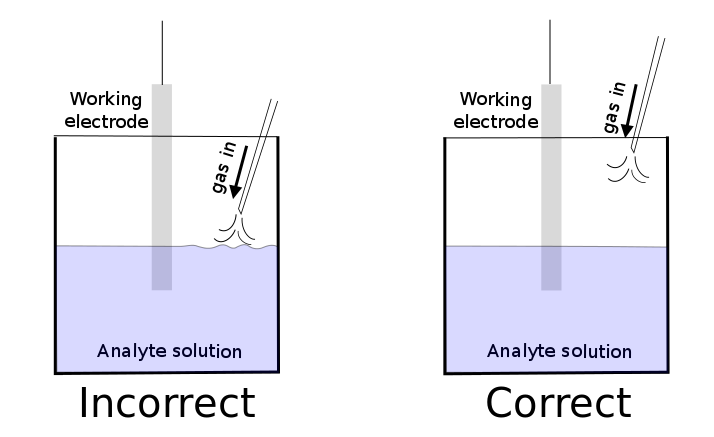
hover over pic for description



Did I earn one of these yet?

is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.