What Is Cyclic Voltammetry?
Before we define cyclic voltammetry, what is voltammetry? For our purposes, voltammetry is any experiment where we expose a solution of an analyte to an electrode, change the electrode potential, and observe the current that flows in response.
In other words, we make a circuit out of a solution containing a molecule that we are interested in studying (analyte), change the voltage (electrode potential) on one of the electrodes, and see what voltage is required to transfer electrons (cause current to flow) between the electrode and our molecule of interest. Incorporating our analyte into a circuit is as easy as dipping two electrodes into our analyte solution. These two electrodes are connected to a potentiostat, which is an instrument that controls the voltage (potential) of each electrode. Voltammetry is a sensitive analytic technique that informs us about the thermodynamics and kinetics of electron transfer for a given analyte.

hover over pic for description
If we were to only sweep (change the electrode potential) in one direction and stop, the experiment would be referred to as a “linear sweep voltammogram,” but if we tell the potential sweep to reverse directions when the potential reaches a certain value and return to the starting potential, we are now performing “cyclic” voltammetry and collecting a “cyclic voltammogram,” or “CV”. We refer to the range of potentials that are accessed while collecting a CV as the potential window.
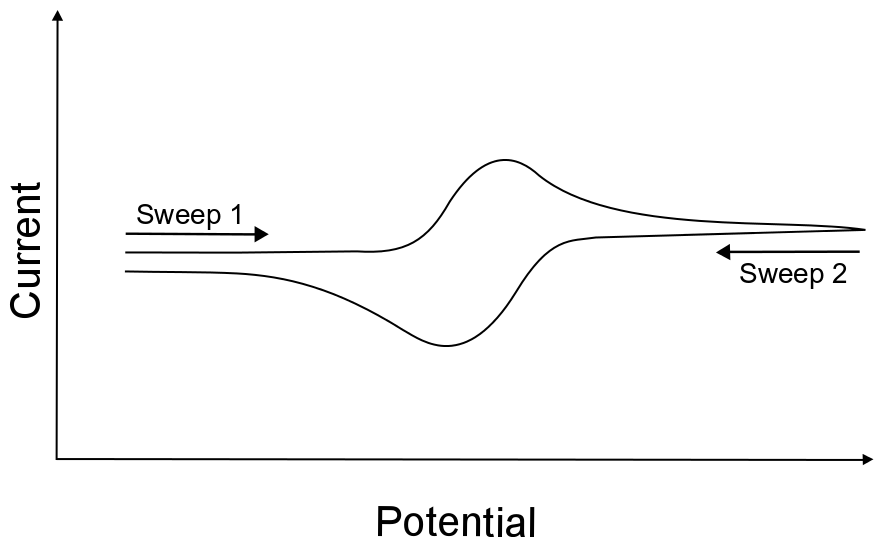
hover over pic for description
In chemistry, we should be comfortable with thinking about the change in the potential energy of a molecule during molecular transformations in the context of acid dissociation, ligand binding, or conformational changes. We should also have no problem comprehending that a given molecule has a different potential energy than an identical molecule to which one electron has been added. We might also be familiar with the concept of band theory, which describes the potential energy of electrons within solids. In fact, electrode potential can be thought of as the potential energy of electrons in the electrode. Most electron transfers can be understood by thinking about the potential energies of the analyte and the electrons in the electrode.
If our analyte is redox–active, it will exchange an electron with an electrode when it reaches certain potentials. Our redox–active analyte will undergo reduction (accept an electron from the electrode) when the potential energy of the electrons in the electrode is higher than the potential energy of the empty molecular orbital on the analyte, so it is energetically favorable for an electron to transfer from the electrode to the analyte. Conversely, our analyte will undergo oxidation (losing an electron to the electrode) when the highest energy electron in the molecule is at a higher potential energy than the electrons in the electrode, and it becomes energetically favorable for it to transfer from the molecule to the electrode. In both of these cases, electrons are transferred in order to minimize the potential energy of the total system. We can use the potentiostat to change the electrode potential and observe where we see current flowing in order to learn about the electron transfer energetics of our analyte.
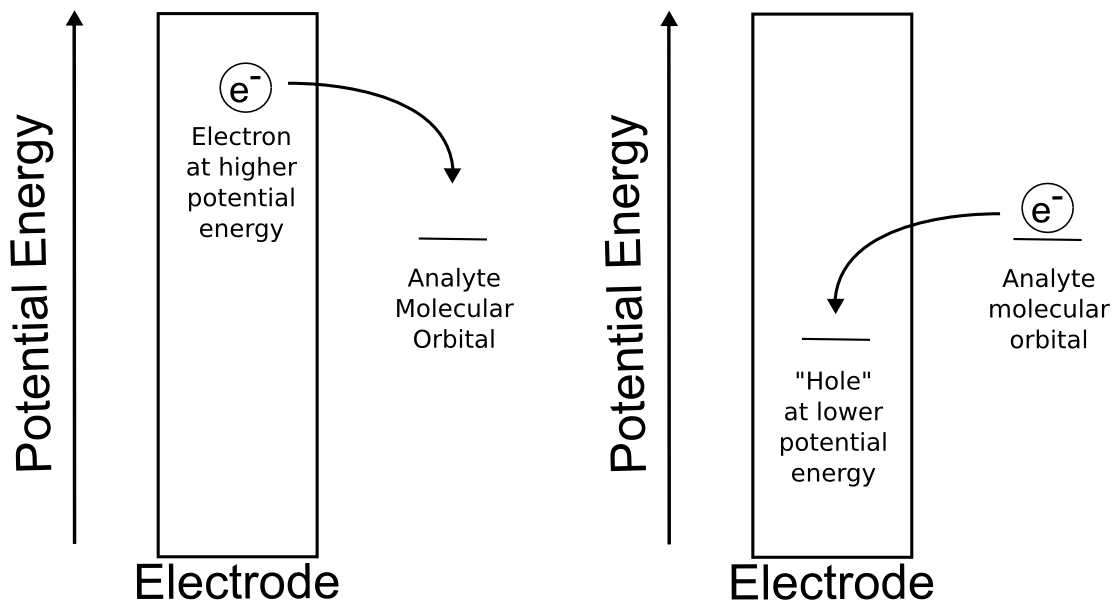
hover over pic for description
In addition to studying the energetic landscape of analyte, we can get a sense for the stability of the molecule that results from an electron transfer between the electrode and the analyte by cycling the potential at different rates. These are the underlying concepts that are responsible for the appearance of CVs and they will be explained further in the following chapters, but hopefully this short introduction is sufficient for understanding what is happening when a CV is collected, and why it is important to avoid anything that can interfere with electron transfer between the electrode and the analyte. The rest of this chapter and the next chapter will detail all components used in a simple cyclic voltammetry experiment, how to prepare them, and how to maintain them to ensure that you avoid contamination or deterioration and collect quality CVs.



Did I earn one of these yet?

is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.