Cell Setup
Concentration of the Electrolyte
First, the electrolyte salt is dissolved in the solvent of choice and this solution is used to dissolve a known amount of analyte. This final solution with a known concentration of analyte is then placed in the cell. The concentration of the electrolyte in the solution can be tailored for a specific experiment; it is most commonly 0.1 M, but can be as high as the solvent will tolerate. A high electrolyte concentration ensures that there is minimal solution resistance, which is an important factor to take into account before starting an experiment. In certain low polarity solvents, like tetraglyme (TEGDME), dimethoxyethane (DME), or tetrahydrofuran (THF) higher concentrations of electrolyte salt are often required. For non-aqueous experiments, an aliquot of the electrolyte solution can be used to make the reference compartment solution.
Concentration of the Analyte
Nearly all meaningful interpretations of CVs require the knowledge of the solution concentration of the analyte. Analyte concentrations usually range from 0.1 – 10 mM, but they can be adjusted according to your needs, 0.5 – 1 mM are common concentrations.
Cell Setup
After the electrolyte solution is prepared, the analyte is added and the resulting solution is placed in the cell. If the analyte is a gas, sparging the electrolyte solution in the cell for 2 – 20 minutes (depending on the volume of the solution) is sufficient to saturate it; a blanket of the gas should be maintained above the solution for the duration of the experiment to ensure that no gas from outside the cell makes its way inside. If a volatile solvent is used, the gas should be run through a gas sparger first in order to pre-saturate the gas and prevent unwanted evaporation.
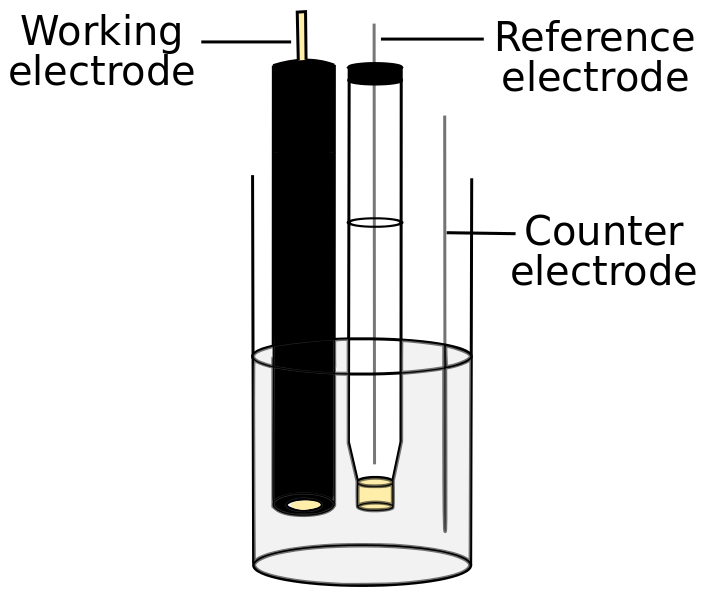
Electrode Placement
The working electrode should be suspended in solution so that the active electrode surface is far away from the solution surface and the edges of the cell. The reference electrode should be placed close to the working electrode, but it should not block the working electrode surface. The counter electrode can be placed anywhere in solution. Some commercially available cells come with cell caps that have holes to conveniently hold all three electrodes in place. If the cell has more than one compartment, the working electrode and reference electrode are ideally placed in the same compartment, but the reference electrode can be placed in a separate compartment if one compensates for the solution resistance using the potentiostat (discussed in the next section).
hover over pic for description



Did I earn one of these yet?

is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.