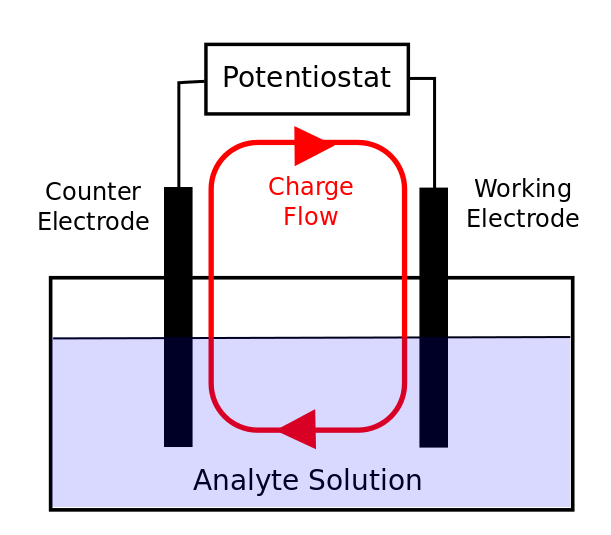
The Electrodes
In most cyclic voltammetry experiments, there are three electrodes. Understanding the role of each electrode will aid in correct preparation of the experiment. The electrodes can be thought about in the context of a simple circuit with resistors and capacitors, so you should treat it like a circuit and make sure that no two electrodes (or the leads connecting them) touch to cause a short circuit!
hover over pic for description
The Working Electrode (WE)
The working electrode is where the action happens, the current that passes to or from this electrode is what is recorded by the potentiostat. For simple cyclic voltammograms, it is helpful to know to the area of the working electrode, which can come in a variety of shapes, but it is usually a flat surface. In order to make sure that the geometric area of the electrode is close to the true surface area, one needs to make sure that the electrode is well polished so there are no jagged edges or grooves. Polishing also ensures that there are no undesired species on the electrode surface that may inhibit electron transfer to or from the electrode. Exposing a polished carbon electrode to the atmosphere for a few hours can negate the polishing and have a noticeable inhibitive effect on its ability to transfer electrons. Even during the course of an experiment, it is clear to a trained eye when the surface of the electrode is changing and affecting the CV. It is important to always check whether CVs are reproducible by occasionally collecting the same CV with the same parameters twice; if the CVs are identical then one can assume the integrity of the working electrode surface is acceptable, but if there is a marked difference in the current traces, there might be some decomposition product that is coating or altering the electrode surface. Common methods of polishing are described in detail in the next section.



hover over pic for description
The Counter Electrode (CE)
The counter (or auxiliary) electrode has the simple requirement of transferring electrons between itself and some species in solution (sometimes even the solvent itself). The current that is measured by the potentiostat is actually the current that flows from the working to the counter electrode, and the WE/solution/CE system can be thought of as a complete circuit through which charge is passed. The counter electrode has to be able to transfer electrons (to or from molecules in solution) quickly in order to prevent any inhibition of the electron transfer at the working electrode. For every electron that is transferred from the working electrode to a molecule in solution, an electron needs to be taken from a molecule in solution by the counter electrode (far away from the working electrode) in order to maintain a balance of charge in solution. Because of the necessity for fast electron transfer at the counter electrode, it is commonly some form or platinum metal, which can easily transfer electrons to or from species in solution. While a platinum wire is more than adequate for most CVs, for experiments where a lot of current (> 1 mA) is expected to flow from the working electrode to or from solution, a high surface area counter electrode, such as platinum gauze or a metal foam, should be used to ensure that the appropriate amount of charge is passed by the counter electrode.



hover over pic for description
The Reference Electrode (RE)
The reference electrode is also connected to the working electrode through the potentiostat, but the potentiostat limits the amount of current that can pass between the two electrodes to nearly zero. As its name suggests, the reference electrode is the electrode to which the potential of the working electrode is referenced. Some reference electrodes consist of redox systems for which the absolute potential is known, but the only real requirement of a reference electrode is that its potential remains constant for the duration of the experiment. Common reference electrodes are Ag/AgCl, Ag/Ag+ (Ag wire in a solution of 10 mM of Ag+), and saturated calomel electrode (SCE, Hg/Hg2Cl2/sat KCl). Conversion from one reference electrode to another or to the standard hydrogen electrode (SHE, H+/H2) is just a matter of addition or subtraction, as most reference electrode potentials are known relative to each other.

hover over pic for description
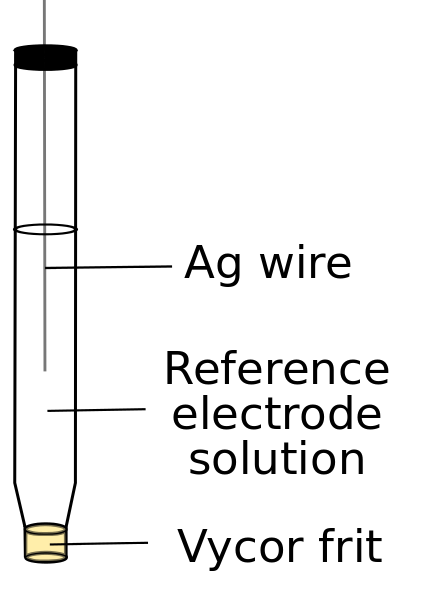
While it is theoretically possible to use Ag wire dipped directly into the analyte solution as a reference electrode for the simplest of CVs, it should be avoided because the slow loss of Ag+ ions may interact with the analyte and any changes in the electrolyte solution (from added substrate, for example) may change the reference potential measured at the Ag wire. The best practice is to isolate the reference electrode from the analyte solution using a vycor (porous glass) frit, which maintains electrical contact while minimizing solution mixing.
Care must be taken to prevent the vycor frit from drying out, which causes the electrolyte salt to crystallize in the pores and render it unusable. The integrity of a vycor frit can be tested by attempting to squeeze liquid through it using a pipette bulb; if fluid filters though easily, the vycor frit should be replaced. Commercially available aqueous Ag/AgCl reference electrodes should be stored in the dark and submerged in solutions that are identical to the solution inside the reference electrode, usually saturated KCl. “Old” Ag/AgCl electrodes can develop a white buildup on the wire, and may drift away from their advertised reference potential. It is best to use a reference compartment solution that contains the same solvent as the electrolyte salt concentration to avoid complications and contamination, so aqueous reference electrodes should not be used for non-aqueous experiments.
For non-aqueous electrochemical experiments, a reference electrode can be made easily from commercially available (or recycled) glass reference electrode compartments, vycor frits, and silver wire. As in the case of aqueous reference electrodes, the silver wire should be submerged in a solution of the same solvent that has the same concentration of electrolyte salt (preferably using the same salt) as the solution containing the analyte. Commonly constructed Ag/Ag+ electrodes for non-aqueous electrochemistry contain a Ag salt (AgNO3, AgBF4, AgPF6), however, faulty vycor frits or sloppy experimental technique can result in the introduction of Ag+ into the electrolyte, which can plate the electrode upon reduction and ruin experiments. One should also be cautious of making Ag+ solutions in DMF, as Ag metal begins to plate out over hours or days, this can be overcome with the addition of an excess of Kryptofix 2.2.2.
hover over pic for description
An alternative to using Ag+ solutions is the use of a silver wire pseudo-reference electrode, which is the same as an Ag/Ag+ electrode except that it doesn’t include any Ag+ in the reference compartment solution. The potential of the Ag wire pseudo-reference electrode is not important, its sole purpose of the pseudo-reference electrode is to remain at a constant potential throughout the experiment. When the researcher is satisfied with the CVs of the analyte, a redox active molecule with a known reduction potential (usually ferrocene, decamethylferrocene, or cobaltocene) is added to the solution as an internal standard and its reduction potential is measured relative to the pseudo-reference potential. All CVs are then adjusted accordingly by subtracting the reference molecule’s reduction potential from all CVs, essentially shifting each CV so that “0” on the x-axis is now the reduction potential of the internal standard. The reduction potentials of many commonly used internal standards are known in various solvents and referenced to various reference electrodes, allowing for the cross-referencing. Note that whenever Ag wire is used, it should be “fresh,” using fine sandpaper is sufficient to remove any surface oxides if the Ag wire has been exposed to air for long periods of time.



Did I earn one of these yet?

is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.