Testing For Adsorption
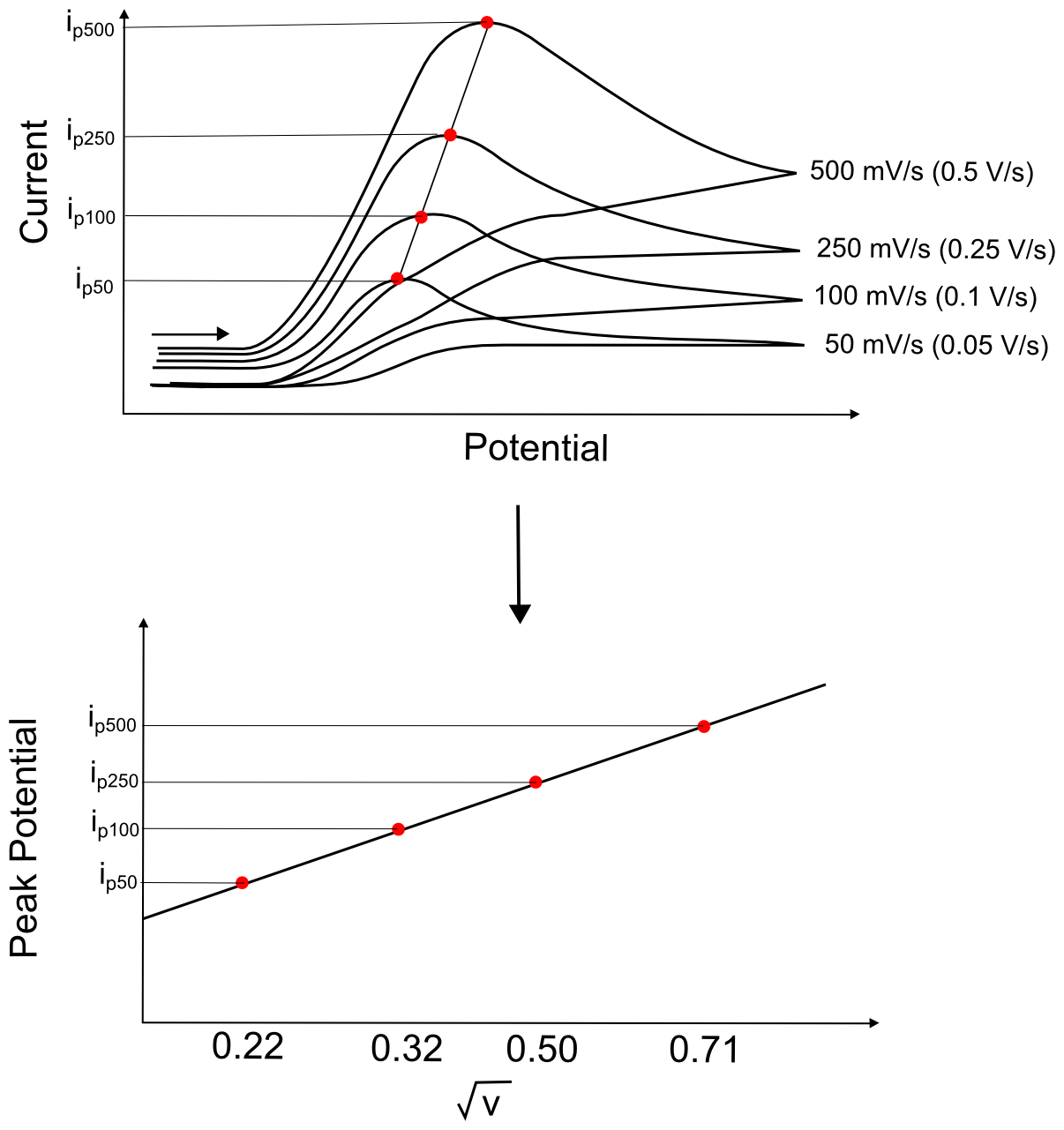
It is fairly easy to check whether the electron transfer takes place between the electrode and a molecule in solution or a molecule that has been adsorbed on the electrode. First, CVs are collected at various scan rates and the peak currents are recorded. Then, the peak currents are plotted vs. the square root of the scan rate. If there is a good linear fit, then the electron transfer is most likely taking place in a truly homogeneous fashion. If the electron transfer takes place in a heterogeneous fashion, where the analyte adsorbs onto the electrode prior to electron transfer, there will be a linear fit on a graph of peak current and the scan rate, as opposed to the square root of the scan rate.
hover over pic for description



Did I earn one of these yet?

is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.