Pseudo-reference Referencing
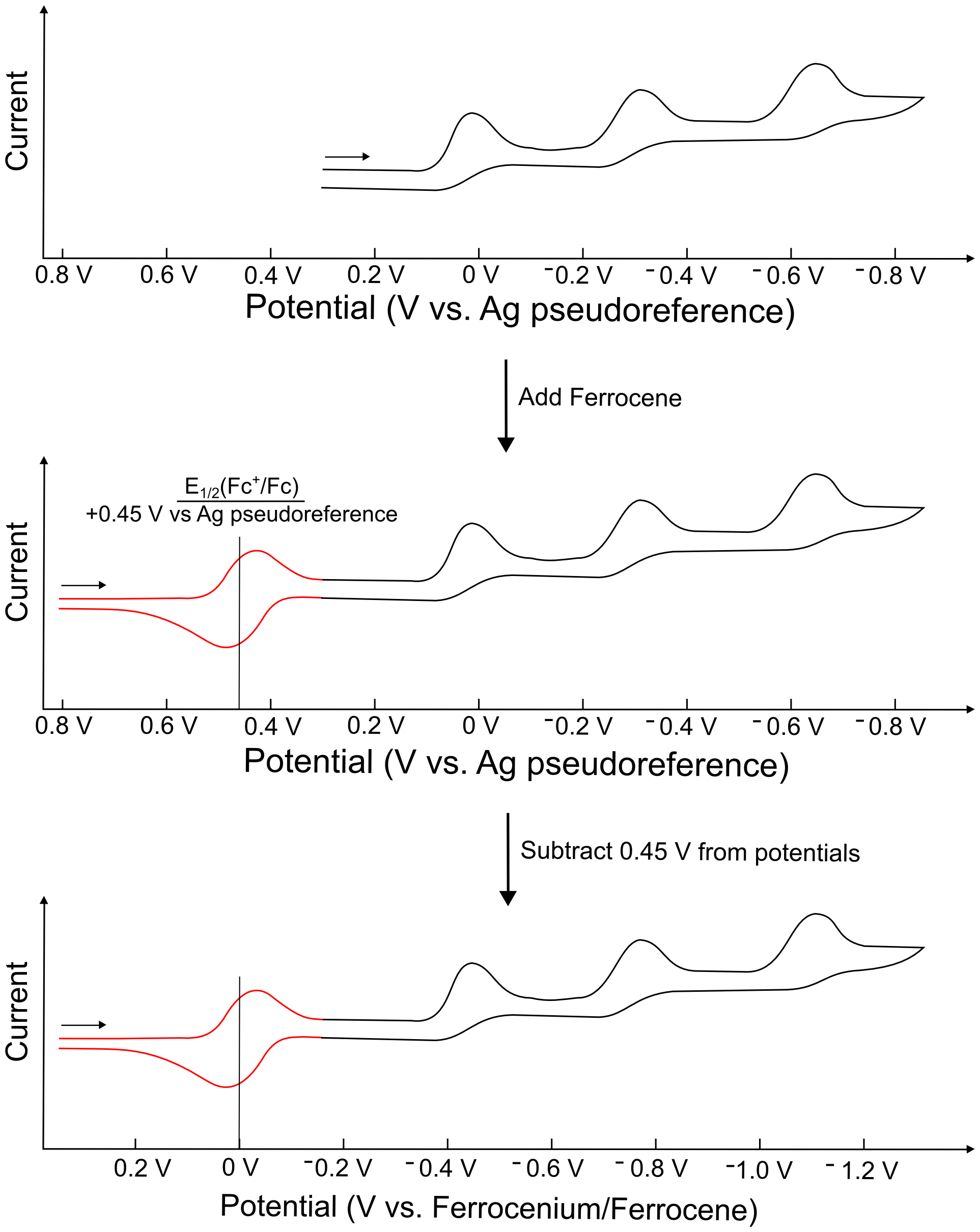
If a pseudo–reference electrode is being used (and is trustworthy), the reduction potential of the internal standard (most commonly ferrocene) should be measured at the very end. Alternatively, if it is known that the presence of ferrocene will not interfere with any of the electrochemical processes and does not overlap with any other features in the CV, the most trustworthy method of referencing the CVs is to add a small amount of internal standard before any CVs are collected, so that the E1/2 of the internal standard can be recorded with every CV.
After the E1/2 of the internal standard is known, that value can be used to readjust the potential scale of the CV. Usually, this readjustment is only a few hundred millivolts, and the resulting potential scale should be labelled "Potential vs. Ferrocenium/Ferrocene" (if ferrocene is the internal reference). If the absolute value of the E1/2 of ferrocene vs. a standard hydrogen, calomel, or Ag/Ag+ is known for the specific solvent used, one can reference their pseudoreferenced CVs to different standard electrodes, if one wishes.
hover over pic for description



Did I earn one of these yet?

is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.