Investigating Individual Waves
Sometimes there are multiple features in a CV, and the apparent reversibility of some waves depend on the reversibility of the other waves. In other words, if the product of the reduction creates an unstable species that falls apart, all subsequent electron transfers will be affected.
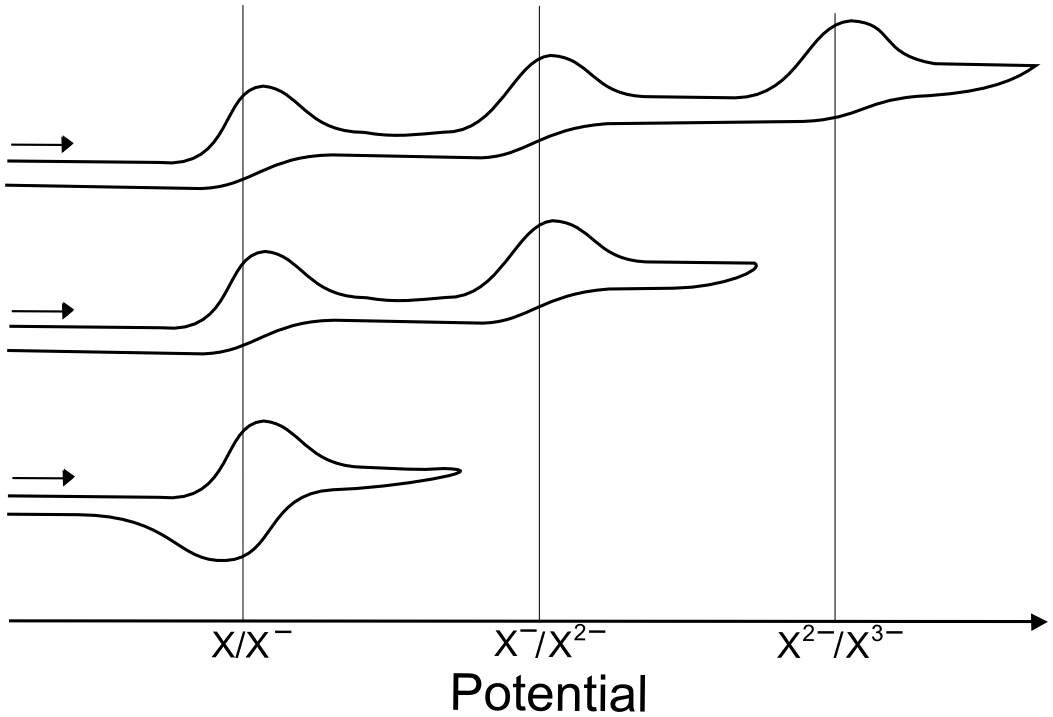
For example, when scanning negatively, if an analyte X has three reduction events, but no return oxidation waves are observed, it doesn't necessarily mean that all three of the reductions are electrochemically or chemically irreversible. It might be due to the fact that one or more of the observed reductions are followed by an irreversible chemical step or decomposition. In our example, a return wave for the X/X– couple is only observed when there is a population of X– to be recollected at the electrode, but if X2– or X3– are not stable in solution, there will not be much X– left for the return scan. Another possibility is that the decomposition coats the electrode and inhibits electron transfers for the rest of the scan.
hover over pic for description
One way to test if this is the case is to cut the scan short and reverse the direction before one of the reductions takes place. In the case above, the scans can be cut short before the third reduction, which will result in one of two possible outcomes; either some of the waves become reversible or the waves remain irreversible. The same procedure can be applied to isolation of the first reduction wave by stopping the scan before the second reduction event, in the case above, the first wave is reversible, but an event that results from further reductions prevents it from looking that way on the return scan.



Did I earn one of these yet?

is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.